Photo by Nappy on Unsplash
FDA Warns of Patients Overdosing Compound Versions of Injectable Weight-Loss Drugs
July 30, 2024
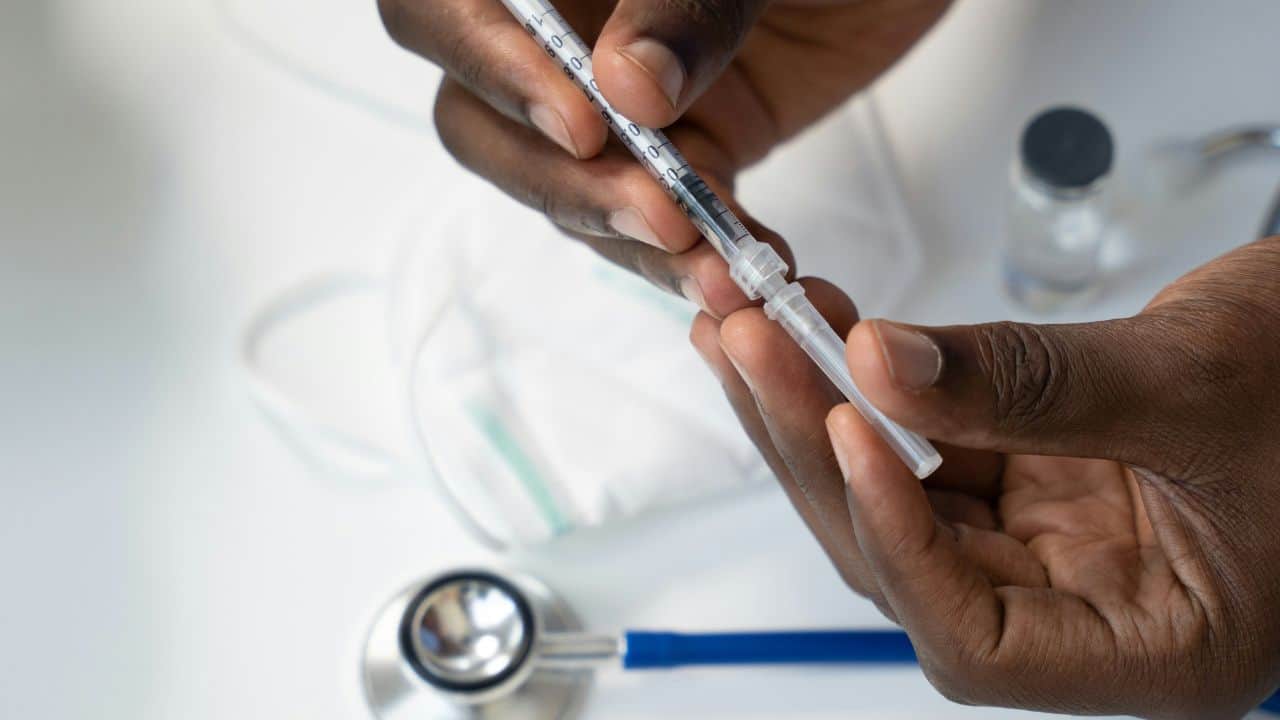
The FDA has issued a warning that some patients are overdosing themselves with compounded versions of injectable weight-loss drugs. Patients are reportedly injecting too much per dose, therefore causing serious health complications.
In an FDA alert, the health organization states the issues stem from patient errors in measuring and self-administering compound versions of drugs containing semaglutide. The report states, “Many of the patients who received vials of compounded semaglutide lacked experience with self-injections, according to the adverse event reports. Unfamiliarity with withdrawing medication from a vial into a syringe and coupled with confusion between different units of measurement (e.g., milliliters, milligrams, and ‘units’) may have contributed to dosing errors.”
The statement continues, “FDA encourages health care providers and compounders to provide patients with the appropriate syringe size for the intended dose and counsel patients on how to measure the intended dose using the syringe.”
The FDA says patients may be overdosing on imitation semaglutide weight-loss drugs because the directions are unclear https://t.co/Z4FfijIGUT
— Insider (@thisisinsider) July 27, 2024
The FDA states that for some patients, the recommended dose of the drug they purchased to assist in their weight loss was unclear. The health organization cited one patient who used a telemedicine provider as a prescriber. However, after not being able to receive a clear answer as to how much of the injectable to take, the patient searched online for answers and ended up taking five times the intended dose.
In December 2023, CNN reported that poison control centers noticed increased calls related to the dosing of these medications. Patients experienced adverse reactions, leading them to seek assistance.
CNN reported that compounded versions of semaglutide are often different from the drugs approved by the FDA. Some compounded drugs on the market contain semaglutide salts called semaglutide sodium and semaglutide acetate, which have not been tested and approved to be safe by the FDA. Often, these compounded versions are sold in unapproved dosages.
Semaglutide is the active ingredient in three FDA-approved medications: Wegovy, Ozempic, and Rybelsus. According to the FDA, typical patient reactions include nausea, vomiting, abdominal pain, fainting, headache, migraine, dehydration, acute pancreatitis, and gallstones.
Recent News
Delta Seeks Outage Damages From Microsoft, CrowdStrike
The airline plans to sue both Microsoft and CrowdStrike for damages.
Sprouts Shares Positive Q2 Financial Results
Sprouts Farmers Market, Inc. reported robust second-quarter results ending on June 30, 2024.
Johnnie Walker Maker, Diageo, Posts Largest Sales Drop Since the Pandemic
As inflation and high interest rates force many to find ways to cut spending, it appears alcohol is also losing its buzz.
IKEA Focuses on Sleepeasy With New Pop-Up Event
IKEA U.S. is making new strides in the furniture retail market by launching The IKEA Sleepeasy, an immersive pop-up event that will take place in New York in August.
